Propionic Acidemia Foundation awards $50,000 Continuation Grant
ATP further inhibits propionyl-CoA carboxylation according to our recent ischemia study. The impaired energy metabolism and propionyl-CoA accumulation forms a vicious circle.
JOURNEY OF MUHAMMAD WASIQ
(DoB: 17 May, 2019) – Islamabad, Pakistan
Metabolic Disorder – Propionic Acidemia
Muhammad Wasiq born on 17 May 2019in capital of Pakistan in an economically strong family. After few initial days care in one of the most equipped private hospital in Pakistan, he began to grow well without any complication. Wasiq was active, happy and a healthy child and his parents used all best parenting practices learning from his elder two brothers’ brought up specially in diet. Wasiq was active than any other child at the age of seventh months.
 At seventh month, Wasiq suffered high fever for continuous eight days after which his health started to decline. In those months, he also had mild constipation at random intervals. Days came when his constipation and vomiting extended to a spell of eight days. Medical tests verified Propionic Acidemia (PA) at 12th Month. These tests are done in Pakistan in only one hospital at Karachi city. After that, every day turned out to be a challenge for the family.
At seventh month, Wasiq suffered high fever for continuous eight days after which his health started to decline. In those months, he also had mild constipation at random intervals. Days came when his constipation and vomiting extended to a spell of eight days. Medical tests verified Propionic Acidemia (PA) at 12th Month. These tests are done in Pakistan in only one hospital at Karachi city. After that, every day turned out to be a challenge for the family.
Pakistan is a country where pre marriages medical genetic tests are discouraged. Health budget is not enough to respond to national needs. Private medical facilities are preferred over Government services. Screening and by birth medical tests facilities are not available in country. As a result, there are multiple medical issues spread out in country.COVID-19 had devastating impact on medical and economic conditions in country with complete lock downs and situation further deteriorated.
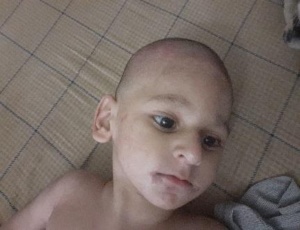 When Wasiq was diagnosed with PA, we were informed about the damage already done in brain by protein intake. He could not sit, speak and had wavy body movement. We needed medical advice but due to the fact that PA cases are very rare in Pakistan, we struggled to seek medical advices despite of reaching out to best hospitals in country. Until one day, we found a doctor who is the only expert in metabolic disorders in country. After couple of advices through virtual meetings, we travelled to Karachi to meet her face to face. Doctor adjusted our diet with medicines to control ammonia level. We controlled his diet and gave medicines. Soon in this phase, Wasiq started to show rashes on face and on his back which was clear indication of protein deficiency. The problem was that we were following and implementing doctor’s advised plan but struggled to achieve nutritional diet balance and we needed further advice.
When Wasiq was diagnosed with PA, we were informed about the damage already done in brain by protein intake. He could not sit, speak and had wavy body movement. We needed medical advice but due to the fact that PA cases are very rare in Pakistan, we struggled to seek medical advices despite of reaching out to best hospitals in country. Until one day, we found a doctor who is the only expert in metabolic disorders in country. After couple of advices through virtual meetings, we travelled to Karachi to meet her face to face. Doctor adjusted our diet with medicines to control ammonia level. We controlled his diet and gave medicines. Soon in this phase, Wasiq started to show rashes on face and on his back which was clear indication of protein deficiency. The problem was that we were following and implementing doctor’s advised plan but struggled to achieve nutritional diet balance and we needed further advice.
On extensive search to solve this issue, we connected with a nutritionist who was keen to connect and had interest in metabolic probes. We shared the diet plan and medicine package with nutritional expert who readjusted the whole package and we started to see improvement in child condition. Wasiq was much stable now and now came the rehabilitation phase by assessing his complete body functions. Emphasis focused on physiotherapy, occupational and speech therapy with further confirmation of hearing loss. He can now sit with support and can move his feet with support if brought in standing posture however the backbone imbalance and jerky movement does not help much to stable him.
Present challenge is to find food and medicines which are often not available in city. Searching, tracing, order and delivery from other city take time and resources. As we know PA child follow vegan diet line but it also needs nutrients to bring diet balance, substitute formulas are hard to find. There is only one importer in country and because data of such children is not available, need & supply balance is always out. Consequently, every day is a challenge.
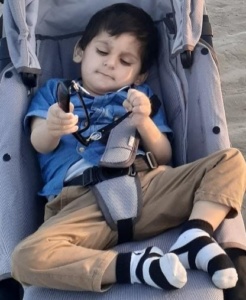 There is allot to be done for such children. Families of metabolic disorders have now connected through social media to help each other and extend help to meet daily needs. Country like Pakistan where infrastructure is not strong, help comes through social support of inter connected communities through welfare initiatives. Children like Wasiq are extending help to other needy children on weekly bases by sharing food and by supporting financially. The issue is taken up collectively by parents to government authorities and escalated on local media. Substantial action is awaited. Every day passes with a hope that the voices will be heard soon to get consistent support.
There is allot to be done for such children. Families of metabolic disorders have now connected through social media to help each other and extend help to meet daily needs. Country like Pakistan where infrastructure is not strong, help comes through social support of inter connected communities through welfare initiatives. Children like Wasiq are extending help to other needy children on weekly bases by sharing food and by supporting financially. The issue is taken up collectively by parents to government authorities and escalated on local media. Substantial action is awaited. Every day passes with a hope that the voices will be heard soon to get consistent support.
Written on 2nd May, 2021 by father Saqib Javed, Pakistan
PAF Awards $49,953 New Research Grant in 2021
PI: Pawel Swietach, Professor of Physiology, Department of Physiology, Anatomy & Genetics, University of Oxford, England
“Aberrant protein propionylation and distinct histone marks in propionic acidemia: new disease mechanisms and risk factors for cardiac disease”
The challenge placed on our hearts – to contract and relax in a correct sequence and with adequate strength – is formidable. The elegant biological solution to this mechanical problem is an organ that pumps millions of liters of blood to support life for many decades. However, the quality and span of a person’s life is strongly linked to cardiac health. Thanks to scientific breakthroughs, better treatments are now available for cardiac disease, allowing patients to live longer and happier lives. Our goal at Oxford University’s British Heart Foundation Centre of Research Excellence is to ensure that scientific progress addresses a wide spectrum of disorders, irrespective of their incidence.
Cardiac problems are common in propionic acidemia (PA). Sadly, dilated cardiomyopathy and long-QT syndrome are often the cause of childhood death. In order to treat and prevent these cardiac problems, we must first understand the underlying mechanisms. Once these processes are described, our aimis to identify targets for drugs or interventions. We believe that this ambition is achievable thanks to the wealth of knowledge about the heart and the vast repertoire of drugs approved for therapy in various other cardiac conditions. Many of these drugs could be “repurposed” for PA-associated disorders, giving hope to many families for a timely treatment.
For this PAF-funded project, we have assembled a consortium of scientists who are eager to devote their expertise to studying PA. My laboratory’s expertise is in cardiac cellular physiology in the context of acid-base disorders. We are joined by Tom Milne who is Associate Professor in Epigenetics at Oxford, Holger Kramer, an expert on proteomics, and Steve Krywawych, principal biochemist at Great Ormond Street Hospital in London. Resources and facilities made available to this project include a mouse model of PA, courtesy of Michael Barry and Lourdes Desviat, methods to characterise cardiac function from the cell to organ level, as well as measurements of changes at the protein and gene level. This interdisciplinary but focused approach allows us to identify potential targets for PA treatment. Indeed, our preliminary findings point to one such enzyme, and the aim of this project is to test and validate our hypothesis.
PA is associated with major metabolic changes, and many of these substances are not merely intermediates in a chain of events, but can have strong biological actions that are not always intuitive to predict. Our project will investigate how the build-up of propionate affects cardiac genes through a chemical reaction that causes DNA scaffolds (called histones) to “open up” genes that should not normally be expressed in a healthy heart. Many genes will be affected by this, but some are more closely linked to the cardiac disorder. After identifying these lead genes, we will test the extent to which blocking these could be curative. In parallel, we will investigate if propionate can also react with other targets in the cell, such as proteins underpinning contraction. Indeed, our work suggests that a promising avenue for research relates to so-called excitation-contraction coupling, a process that converts cardiac electricity to a mechanical response.
We are excited to be part of the PA research family and wish to take this opportunity to invite patients, carers, and supporters to our lab for a visit.
Update 8/2022 – Final Report
ABERRANT PROTEIN PROPIONYLATION AND DISTINCT HISTONE MARKS IN PROPIONIC ACIDEMIA: NEW DISEASE MECHANISMS AND RISK FACTORS FOR
CARDIAC DISEASE
Final Report – August 2022
PI: Pawel Swietach (Oxford University)
Non-confidential report for dissemination
Patients affected by propionic acidemia (PA) present with disturbances in the levels of metabolites, notably propionate. This small (three-carbon) molecule is normally produced
from the breakdown of substances in the diet, such as branched-chain amino acids and odd-numbered fatty acids. In PA, however, genes responsible for propionate processing are
inactivated by inherited mutations. A long-standing view postulates that the ensuing biochemical milieu is responsible for the dysfunction of multiple organs affected in PA.
Understanding how the heart is affected in PA is particularly important, because many childhood deaths have been linked to cardiac disease. However, the precise mechanism
linking the metabolic disturbance with heart disease in PA is unclear. Without this detailed information, it is difficult to propose new cures and improve disease management before
viable gene therapies are available. Moreover, knowledge of the molecular mechanisms has broader impact on cardiac health, because elevations of propionate have also been
described in other diseases, such as diabetes.
The aim of our PAF project was to investigate how the metabolic derangements in PA affect proteins through so-called post-translational modifications, i.e. chemical ‘editing’
that can affect their functions. Using a mouse model of PA, we showed that histones, the protein scaffold of DNA, undergo two types of modifications in the heart: propionylation and acetylation. We then demonstrated how these actions affect the expression of genes in the heart. Strikingly, we found that several genes, previously implicated in cardiac disease, become aberrantly activated in PA, and we speculate that dampening this PA-driven genetic response may alleviate the pathological changes experienced by patients. Through our observations of the mouse model of PA, we identified a novel biochemical pathway that offers an alternative means of processing excess propionate in the heart. Activation of this pathway was associated with a less severe disease presentation in mice. We hypothesize that this pathway could be exploited therapeutically in PA patients, and our immediate aims for the future are to identify the best approach for exploiting this protective reservoir for propionate in the heart.
In summary, the PAF project has (i) delivered novel mechanistic insights into how propionate affects the heart using state-of-the-art methods in metabolomics, transcriptomics,
chromatin biology, and physiology, and (ii) revealed new pathways for propionate processing that by-pass the mutated enzymes in PA patients.
“Partners in Progress: Families and Scientists Catalyze Research for Rare Diseases”
On Nov. 15, 2017, Baylor College of Medicine and Texas Children’s Hospital hosted a panel discussion as part of theEvenings with Genetics seminar series held at the Children’s Museum of Houston. The topic was “Partners in Progress: Families and Scientists Catalyze Research for Rare Diseases” and panelists traveled from both coasts and the center of the country. Panelists included Jill Chertow Franks, President, Propionic Acidemia Foundation; Cynthia Le Mons, Executive Director of the National Urea Cycle Disorder Foundation, Tracy Smith Hart, Chief Executive Officer, Osteogenesis Imperfecta Foundation and Brendan Lee, MD, PhD, Robert and Janice McNair Endowed Chair, Professor, Department of Molecular and Human Genetics, Baylor College of Medicine. These family/scientist partnerships are a new and exciting development in the research efforts for those impacted by rare diseases.
The audience of almost 80 people consisted of parent leaders, rare disease foundations, medical students, genetic counseling students, pharmaceutical companies and undergraduate biotech majors. Each panelist discussed the partnerships with rare disease organizations and scientists and their strategies for success in obtaining funding for research from the National Institutes of Health (NIH). In addition, panelists shared how they became involved in the rare disease organization and offered advice for other rare disease organizations as well as researchers with regards to working together to submit requests for funding. Dr. Brendan Lee discussed the positive impact of family/scientist partnerships and that these collaborations highly beneficial for progress in understanding rare disorders and developing effective therapies.
Susan D. Fernbach, RN, BSN
Director of Genetic Outreach
Director of Diversity and Community Engagement
Assistant Professor, Dept. Molecular and Human Genetics
Baylor College of Medicine/Texas Children’s Hospital